In the past 10 to 15 years, biologically active agents with antigen binding activity, like antibodies, antibody fragments, antibody-like molecules, and scaffold proteins, have gained significant relevance. However, their therapeutic use has yet been limited because of rapid renal excretion, poor solubility, immunogenicity, reduced binding affinity, and/or avidity as compared with native human antibodies.
For this reason, many attempts have been made to improve the pharmacological properties of such antigen binding proteins ordinarily having molecular weights far below 50,000 Dalton. Reviews have been published in Nature Biotechnology Volume 21, Number 4, 2006: 1126-36 or Nature Reviews Immunology, Vol 6, 2006: 343 -357.
celares GmbH and Scil Proteins GmbH have co-developed a star-shape binding agent for the multimerization of antigen binding proteins. This reagent, named celaSTAR, is characterized by a defined structure and adjustable linker length. With celaSTAR, it is possible to prepare conjugates with decelerated renal clearance showing an extraordinary high avidity. Thus, the binding constants of the tetramer and monomer differ by almost two orders of magnitude, which is much more than the sum of binding in the tetramer (i.e., a true multivalent effect).
An example is a TNF-α binding affilin®. Tetramerization with celaSTAR it has been able to increase its affinity towards TNF-α in an in-vitro assay 30-fold.
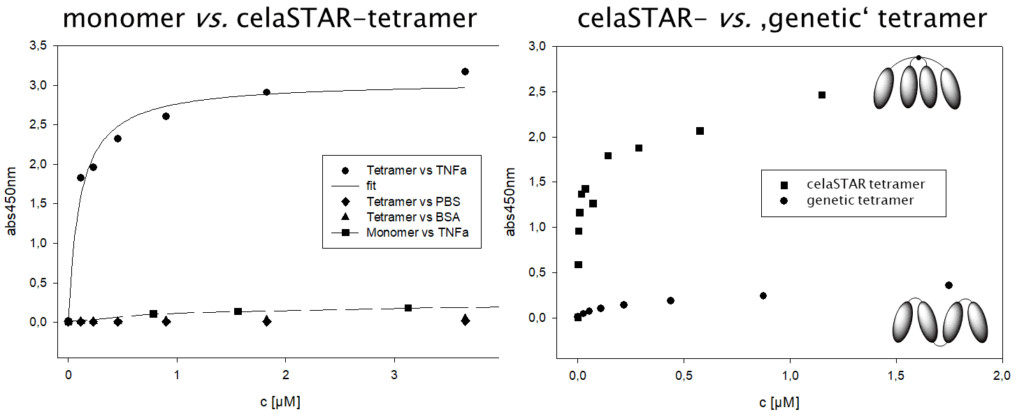
Some advantages of celaSTAR multimers versus genetically engineered tetramers are shown in the table below.
| Genetic multimerization | celaSTAR multimerization |
|---|---|
| Established technology | Single compound conjugate, simple high-yielding chemistry |
| Restricted to linear multimerization | Retaining full activity plus potential for a high MVE |
| Risk of poorly increased activity and avidity (risk of poor MVE) | Flexible attachments possible |
Our multimeric binding agent is covered by the patent portfolio based on WO 2008/096012 A2.
Contact partner

Dr. Sophie Janke
Head of Synthesis
+49 30 9489 2350
